Abstract
Introduction: Solitary plasmacytoma is a malignant collection of plasma cells (PC) in the soft tissue (EP) or skeleton (BP) without multiple myeloma (MM). Treatment is based on excision and/or radiation therapy. A proportion of patients with EP/BP progress to MM. In this study we describe the baseline characteristics/clinical outcomes and create a clinical model of progression in a large cohort of 98 plasmacytoma patients diagnosed between 1986 and 2015.
Methods: We retrospectively searched our PC database from 1986-2015. 218 patients were identified, of whom 120 were excluded due to incorrect diagnosis (n=40)/lack of baseline data (n=78)/duplicate patient (n=2). Summary statistics were given for categorical and continuous variables. Chi-squared and Fisher's exact tests were used to examine the associations between categorical variables and Wilcoxon's rank sum test was used for continuous variables. The primary outcomes were time to myeloma (TTM:time from diagnosis to myeloma progression), progression free survival (PFS:time from diagnosis to myeloma progression or death) and overall survival (OS:time from diagnosis to death of any cause). Kaplan-Meier curves were plotted for PFS/OS/TTM. Univariate cox proportional hazard models were used to determine the effects of potential risk factors (RF) on TTM, PFS and OS. Multi-covariable Cox proportional hazard models were used to identify RF on time to myeloma. A multi-covariable Cox regression model was fitted using backwards variable selection with a p-value of ≤ 0.05 as limit for inclusion. All tests are two-sided. All analyses were conducted using SAS 9.4 (SAS, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) software.
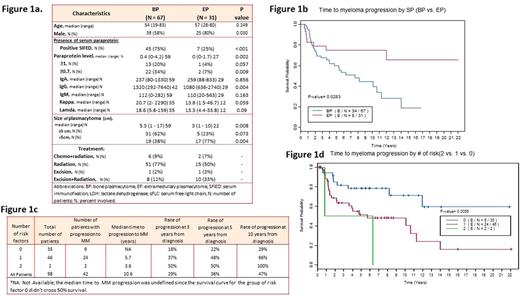
Results: Figure 1a shows baseline patient/treatment characteristics. Most patients (n=88/98; 90%) had advanced imaging (MRI, PETCT, CT scan) and bone marrow biopsy at diagnosis (n=97/98; 99%). The median follow-up time was 7.64 years (range 0.2-23.5) among the 64 alive patients. There were 42 myeloma progressions among the 98 patients. BP (n=34/67; 50%) progressed at a significantly higher rate than EP (n=8/31; 25%; p=0.02) (Figure 1b). On univariate analysis baseline bone plasmacytoma, lower hemoglobin, immunoparesis, presence of blood paraprotein and increased light chains in blood were associated with unfavorable TTM outcome (p<0.05). Advanced age, increased Bence Jones in urine and being male at diagnosis were associated with worse OS (p<0.05). Lower hemoglobin, higher LDH, presence of immunoparesis and blood paraprotein at diagnosis were associated with worse PFS (p<0.05). There were no statistical differences in PFS (p=0.09) and OS (p=0.33) between EP and BP, although there was a trend for improved PFS with EP. There were 18 deaths for BP and 12 deaths for EP. Cause-specific mortality showed that for BP: n=6/18 (33%) died of MM, n=7/18 (39%) had unknown cause of death, n=5/18 (27.8%) died of other malignancies. For EP patients, cause of death was unknown [n=6/12 (50%)], MM [n=4/12 (33%)], bladder cancer [n=1/12 (9%)] or infection [n=1/12 (9%)]. Baseline calcium, hemoglobin, and presence of positive immunofixation, immunoparesis, blood paraprotein, Bence Jones ≥ 50 mg, BP vs EP and tumor size were included in the multi-covariate analysis for TTM. The reduced final model showed that presence of immunoparesis (p=0.004) and blood paraprotein (p=0.02) at baseline were significantly associated with TTM. We then created a model using 0 (no risk factors), 1 (either having baseline immunoparesis or presence of blood paraprotein) or 2 risk factors (having both immunoparesis and blood paraprotein at baseline). The rates of progression to myeloma for all BP/EP patients at 10 years with 0, 1 and 2 risk factors were 29%, 66% and 100% (p=0.006) (Figure 1c/d).
Conclusion: BP and EP are precursors of MM. At a median follow up of 7.64 years, 26% of EP and 51% of BP patients progressed to MM. These rates are similar to those reported for low and intermediate risk smoldering myeloma, emphasizing the need for continued follow up and prevention strategies (i.e., study NCT02516423 of systemic treatment with ixazomib, lenalidomide, dexamethasone, zoledronic acid). Clinical models such as the one presented here may guide clinicians in optimal follow up or inclusion in clinical trials for prevention of progression to MM.
Manasanch: takeda: Consultancy; sanofi: Research Funding; adaptive biotechnologies: Consultancy; merck: Research Funding; celgene: Consultancy; quest diagnostics: Research Funding. Lee: Takeda: Consultancy; Celgene: Consultancy; Pimera Inc: Consultancy; Adaptive: Membership on an entity's Board of Directors or advisory committees; Eutropics Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding. Thomas: Celgene: Research Funding; Bristol Myers Squibb: Research Funding. Patel: Juno: Consultancy; Celgene: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Orlowski: BioTheryX: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal